electron configuration fe2+|Iba pa : Clark Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s . The Taj Mahal (/ ˌ t ɑː dʒ m ə ˈ h ɑː l, ˌ t ɑː ʒ-/ TAHJ mə-HAHL, TAHZH-, Hindi: [taːdʒ ˈmɛɦ(ɛ)l]; lit. ' Crown of the Palace ') is an ivory-white marble mausoleum on the right bank of the river Yamuna in Agra, Uttar Pradesh, India.It was commissioned in 1631 by the fifth Mughal emperor, Shah Jahan (r. 1628–1658) to house the tomb of his beloved wife, .
electron configuration fe2+,Electron Configuration for Iron (Fe, Fe2+, and Fe3+) Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period .Therefore the Calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 .Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .
Therefore the N electron configuration will be 1s 2 2s 2 2p 3. Video: Nitrogen .electron configuration fe2+In order to write the Mg electron configuration we first need to know the .Electron Configuration Notation:-shows the arrangment of electrons around the .Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 .In order to write the Argon electron configuration we first need to know the .

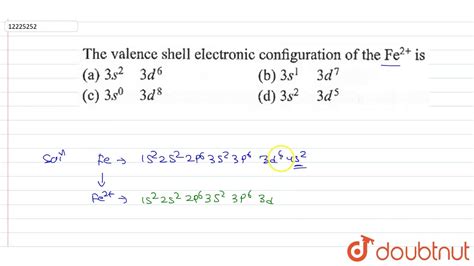
Therefore the O electron configuration will be 1s 2 2s 2 2p 4. Video: Oxygen . Fe2+ electron configuration: 1s2 2s2 2p6 3s2 3p6 3d6. Iron has allotropic forms that exist in different temperatures and is referred to as alpha, beta and gamma iron. Applications and Uses of Iron: Iron is . Electron Configuration of Fe2+ and Fe3+. chemistNATE. 266K subscribers. Subscribed. 5.3K. 705K views 10 years ago. The electron configuration for Fe2+ is 1s2 2s2 2p6 .
The electron configuration of Fe, Fe2+, and Fe3+ can be determined by following the Aufbau principle, Hund’s rule, and the Pauli exclusion principle. Fe has the .Iba pa This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron .
The electronic configuration of Fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and Fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Fe2+ contains 2 fewer electrons compared to the electronic configuration of Fe. Applications of Iron. . This chemistry video tutorial explains how to find the electron configuration of Ions such as Mg2+, P3-, Fe2+, and Fe3+.Electron Configuration - Basic Intro:.
What is the electron configuration and orbital diagram of: Na + P 3– Al 2+ Fe 2+ Sm 3+ Solution. First, write out the electron configuration for each parent atom. We have chosen to show the full, unabbreviated .
electron configuration fe2+ Iba pa What is the electronic configuration of Fe (III)? Solution. Start with the electron configuration of the neutral atom. It is sufficient to use the noble gas .Electron Configuration of Fe2+ and Fe3+ Skip to main content. General Chemistry Start typing, then use the up and down arrows to select an option from the list. . Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and Titanium Ions) Wayne Breslyn. 5961. views. 1. rank. 03:32. El estado fundamental fe3 + la configuración electrónica es 1s2 2s2 2p6 3s2 3p6 3d5. Los primeros 2 electrones se eliminan del orbital 4s porque tiene una energía más alta que el orbital 3d y luego se elimina 1 electrón del orbital 3d, lo que hace que la configuración electrónica de la capa de cenefa sea fe3 + es 3d5.Answer and Explanation: 1. Become a Study.com member to unlock this answer! Create your account. The electron configuration for the iron +2 ion is {eq}1s^2 2s^2 2p^6 3s^2 3p^6 3d^6 {/eq}. The two electrons in the 4s sub .PROBLEM 3.1.13 3.1. 13. Thallium was used as a poison in the Agatha Christie mystery story “The Pale Horse.”. Thallium has two possible cationic forms, +1 and +3. The +1 compounds are the more stable. Write the electron . Hello! So the electron configuration for Fe is [Ar] 3d^6 4s^2. Fe^2+ means that 2 electrons are taken away. You start removing e- from the outermost shell. The outermost shell, in this case, is the 4s orbital. So removing 2 electrons would leave you with the electron configuration of [Ar] 3d^6. Hope this helps!

Quando 2 elétrons são removidos do átomo de fe neutro, o íon fe2+ é formado. O estado fundamental fe2+ configuração eletrônica de fe2+ é 1s2 2s2 2p6 3s2 3p6 3d6. 2 elétrons são removidos do orbital 4s, pois tem energia mais alta que o orbital 3d, fazendo com que a configuração eletrônica da camada de valência de fe2+ seja 3d6.The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).What is the electron configuration for the Fe2+ ion? A) [Ar]4s03d6 B) [Ar]4s23d4 C) [Ar]4803d8 D) [Ar]4s23d8 E) [Ar4s63d2 ; This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer See Answer See Answer done loading. The electronic configuration of Fe is [Ar] 3dX6 4sX2 [ A r] 3 d X 6 4 s X 2 . So after removing two electrons the configuration becomes: [Ar] 3dX6 [ A r] 3 d X 6. But why can't the electrons rearrange themselves to give a more stable [Ar] 3dX5 4sX1 [ A r] 3 d X 5 4 s X 1 configuration? electronic-configuration. transition-metals.Thus, the electron configuration for an Fe2+ ion is 1s22s22p63s23p63d6. Al3+: From Table 2.2, the electron configuration for an atom of aluminum is 1s22s22p63s23p1. In order to become an ion with a plus three charge, it must lose three electrons—in this case two 3s and the one 3p. Thus, the electron configuration for an Al3+ ion is 1s22s22p6.
Die Elektronenkonfiguration von Fe2+- und Fe3+-Ionen wird erhalten, indem Elektronen zunächst aus dem 4s-Orbital und dann aus dem XNUMXs-Orbital entfernt werden das 3D-Orbital. Die Elektronenkonfiguration von Fe, Fe2+ und Fe3+ beeinflusst ihre chemischen Eigenschaften und Reaktivität. auf im Periodensystem.Write the ground state electron configurations of the following transition metal ions. a. Sc3+ b. Cr3+ c. Cu+ d.Au+; Write the ground-state electron configurations of the following transition metal ions. a.Sc^3+ b.Cr^3+ c.Cu^+ d.Au^+ a) Write ground-state electron configuration in complete form (instead of the condensed form) for the Na^+ ion.March 23, 2023. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. This electron must go into the lowest-energy subshell available, the 3s orbital, giving a 1s 2 2s 2 2p 6 3s 1 configuration.Protons, Neutrons, Electrons for Iron (Fe, Fe2+, Fe3+) Iron is a classified transition metal and its symbol is Fe. Iron is the 26th element of the periodic table so its atomic number is 26. . Here, the electron configuration of iron ion(Fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5. This iron ion(Fe 3+) has twenty-six protons, thirty neutrons, and . In this video we’ll use the Periodic table and a few simple rules to find the number of protons and electrons for the Iron (II) and Iron (III) ions (Fe2+ and.
electron configuration fe2+|Iba pa
PH0 · iron 2+ electron configuration
PH1 · how to write electron configuration
PH2 · full electron configuration of platinum
PH3 · fe2+ orbital diagram
PH4 · fe2+ noble gas configuration
PH5 · electron configuration guide
PH6 · electron configuration explained
PH7 · electron configuration chart
PH8 · Iba pa